Introduce
Corrosion has long been the subject of scientific research because of its devastating consequences, and in general, corrosion is a natural phenomenon that can be explained as the decay of a material (usually a metal), or properties that result from a reaction to its environment. The International Organization for International Standards defines corrosion as "a physico-chemical reaction between a metal and its environment that results in a change in the properties of the metal and often in the functional degradation of the metal, the environment, or the scientific system of which it is a part." While common usage often associates corrosion with metal oxidation, oxidation of all forms of natural and synthetic materials, including biomaterials and nanomaterials, is now included in the term corrosion. "Corrosion is an unavoidable interfacial interaction between a material (metal, ceramic, and polymer) and its environment that may result in the consumption or degradation of the material into an environmental component" is a broader and generally accepted alternative definition of corrosion. The environment consists of the entire environment that comes into contact with a substance. Physical state (gas, liquid or solid), chemical composition (composition and concentration) and temperature are the main variables that define the environment.
Cost of corrosion
In the 1960s, the importance of corrosion was recognized when it was recognized that corrosion was damaging the economies of developed countries, that the useful life of manufactured goods was shortened, and that resources were wasted by antimetallurgical processes. In addition, corrosion also involves issues such as human life and safety, huge environmental impact and material protection. The various effects of corrosion are shown in Figure 1-1.
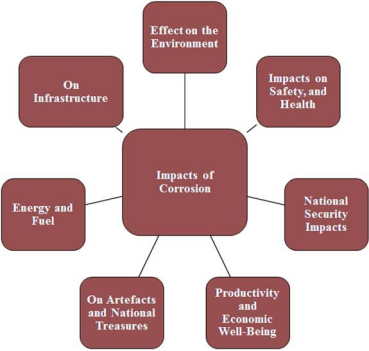
Figure 1-1 Various effects of corrosion.
In order to assess the economic impact of corrosion, some countries have conducted corrosion cost studies. Various factors affecting corrosion cost are shown in Figure 1-2.
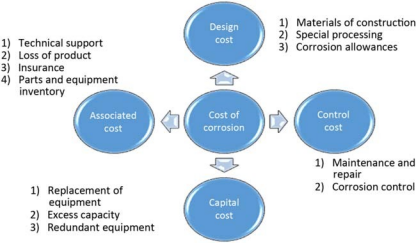
Figure 1-2 Overview of factors affecting corrosion costs.
According to a report published in 2013, Figures 1-3 show the contribution of individual countries to corrosion costs. According to the report, the combined cost of corrosion is 3.4 percent of GDP. The United States contributed 2.7%, India 4.2%, European region 3.8%, Arab World 5.0%, China 4.2%, Russia 4.0%, Japan 1.0%, Asian Tigers 1, Macau 2.5%, and the rest of the world accounted for about 3.4% of the total global corrosion costs.
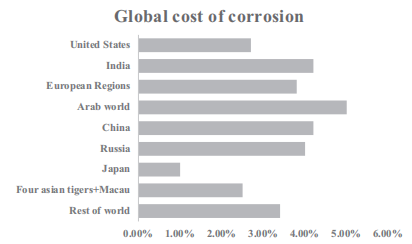
Figure 1-3 Published corrosion costs in 2013 (3.4% of global GDP). GDP, gross domestic product.
Corrosion measuring unit
The corrosion rate can be expressed as an increase (MLS/year)(mpy) per unit of corrosion depth (permeability, MLS/year) or weight loss per unit area in unit area time, usually mdd(mg/cm2 / day) or corrosion current (mA cm22, cm2). Although the preferred SI unit for corrosion rate is mm yr21 or inch yr21, the term mpy is the most commonly used (one mil represents a thousand inches) and the most desirable expression for corrosion rate in the United States, which can be estimated using equation (1).
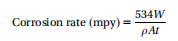
Corrosion type
Corrosion manifests in many forms, and a basic understanding of the details of corrosion types is desirable, as it helps to determine the cause of corrosion and select the most effective method of corrosion protection. In general, classification is based on one of three factors :(1) the properties of the caustic agent, (2) the corrosion mechanism, and (3) the appearance of the corroded metal. According to the appearance, the corrosion type is as follows:
Uniform corrosion or general corrosion
The most common form of corrosion is uniform corrosion. It is often referred to as general corrosion. This corrosion, which occurs more or less uniformly on the entire metal surface exposed to the corrosive medium, is called uniform corrosion. It usually occurs in environments where corrosion rates are relatively low and well controlled (such as chemically treated closed circulation systems and some open water systems). By far, the metals most prone to uniform corrosion are low-alloy iron and magnesium alloys. Total corrosion is caused by local corrosive battery action, that is, multiple anodes and cathodes are active on the metal surface at any given time. Uniform corrosion on bare metal surfaces occurs at almost the same rate. When exposed to open Spaces, soil, and natural waters, cast iron and steel corrodes evenly, leading to rust.
Galvanic corrosion
In electrolyte solutions and electron conduction paths, galvanic corrosion, often referred to as bimetallic or bimetallic corrosion, is the electrochemical behavior of two different metals. This occurs if different metals or alloys with different electrode potentials are electrically connected in a standard electrolyte solution. The anode becomes a more negative or active metal, and the cathode of the electrochemically corroded battery becomes a corrected or precious metal. Among the more expensive materials, the less expensive alloys generally exhibit an increase in corrosion and a decrease or inhibition of corrosion. The potential difference between the two metals, the nature of the environment, the polarization behavior of the metals, and the geometric relationship of the constituent metals all affect the degree of galvanic corrosion.
Pitting
Pitting appears to be the most destructive form of corrosion and is often the cause of plant equipment failure, accounting for about 90% of metal damage caused by corrosion. Pitting is highly localized corrosion of the metal surface without overall corrosion of the surrounding area. A pit can be defined as a cavity whose diameter is equal to or less than the depth of the metal surface. It causes rapid penetration of the metal substrate. It is first a white or grey powdery deposit, similar to dust, that smudges the surface. When the coating is washed off, tiny pits or holes can be seen on the surface. In some cases, pitting can form across the entire metal surface, creating an irregular or rather rough surface profile. Pitting is more difficult to track, prevent, and manage, and is therefore considered more dangerous than uniform corrosion failure.
Crevice corrosion
Crevice corrosion also refers to corrosion in closed areas and is one of the most serious forms of local material degradation. This type of corrosion is comparable to the pitting corrosion that occurs under stagnant electrolysis conditions, while gap corrosion occurs due to local chemical changes, namely the consumption of oxygen in the gap, the rise of pH with the increase of hydrogen ion concentration, and the increase of chloride ion concentration. Oxygen depletion means that the reaction of the cathode to oxygen reduction cannot be sustained within the cracked region, so metal dissolution occurs. Crevice corrosion can occur in any metal and in any corrosive environment.
Intergranular corrosion
Intergranular corrosion, also known as intergranular corrosion, is the localized preferential erosion of grain boundaries or the area around them. Little or no erosion was observed on the main body of the particles. Lack of strength and ductility leads to this form of corrosion. Sometimes, the attack is quick, penetrates deep into the metal and leads to failure. The presence of impurities within the limits, or local enrichment or degradation of one or more alloying elements, is due to this form of corrosion.
Stress corrosion cracking
Structural components subjected to constant tensile stress and corrosive environments may fail prematurely at stresses below yield strength, known as stress corrosion cracking (SCC). Stress may be due to increased load, residual stress from production processes (welding, heat treatment, machining and grinding), or a combination of both.
Erosion corrosion
The term "erosion" refers to decay caused by mechanical forces. When the factors that cause erosion increase the rate at which the metal corrodes, this erosion is called "erosive corrosion." It is the product of a mixture of active chemical environments and high surface flow rates.
Corrosion control method
Corrosion management requires the implementation of engineering principles and techniques to mitigate corrosion to an appropriate degree in the most economical way. To mitigate or control corrosion, different practices can be used
(as shown in Figure 1-4).
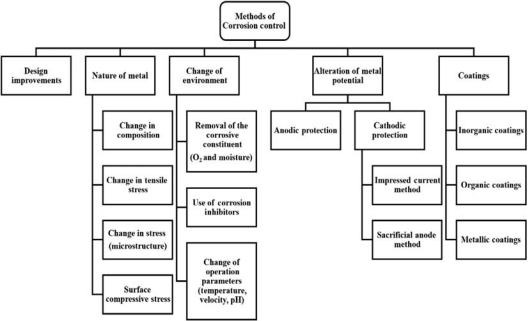
Figure 1-4 An overview of the various methods used in corrosion control.
Correct selection of materials
The method involves the selection and use of materials with high corrosion resistance to increase the life of the structure in a specific environment. While there is no material that can resist all corrosion conditions, it is important to choose the right material to avoid some forms of corrosion failure. For example, titanium is a highly corrosion-resistant material, but it is much more expensive than steel. In addition, it is not as malleable as steel. Carbon steel is the material of choice in oil production systems, especially in equipment such as oil Wells, pipelines, containers and storage tanks, because of its good mechanical properties and low cost. However, options such as stainless steel can be used in situations where more corrosion-resistant materials are needed.
Coatings, linings and non-metallic piping
A coating is a thin material applied in liquid or powder form that adheres firmly and continuously to the material to be protected while curing. For internal use, this method may be referred to as lining. The coating needs to be flexible, resistant to chemical fluid erosion, have strong adhesion, have low or no porosity, and be stable at operating temperatures. The coating is usually used in conjunction with a cathodic protection system to prevent any damage to the coating material. The application of non-metallic pipes is also good in many applications because they do not corrode, but the limitation is that they may be degraded or weakened by environmental erosion.
Factors affecting corrosion rate
There are many internal and external factors that directly or indirectly affect the rate of corrosion. Some of the factors are shown in Figure 1-5. We will focus on external effects, and since other effects cannot be changed, we will
consider their effect on metal corrosion, i.e. the effect of electrolyte solutions on chemical materials.
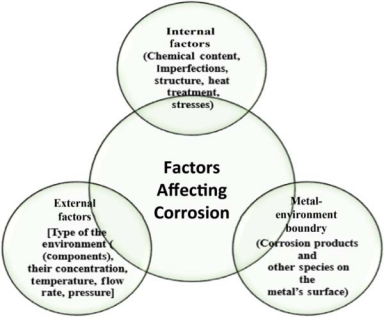
Figure 1-5 General description of the various factors affecting corrosion.
Effect of pH value on metal corrosion
Based on their relationship to pH (corrosion resistance), all metals can be divided into five categories:
1. The corrosion resistance of gold (Au), silver (Ag), platinum (Pt), palladium (Pd), Rhodium (Rh), ruthenium (Ru), mercury (Hg), tantalum (Ta), niobium (Nb), osmium (Os) and iridium (Ir) is not affected by pH value. At pH values of 50 to 14, they are corrosion resistant.
2. Iron (Fe), chromium (Cr) and manganese (Mn) are strongly corroded in acidic solutions above 80°C (pH 4) and very hot alkaline solutions (pH 13.5). They often corrode, but have a low corrosion rate at a neutral pH (6-8) and are not corroded at a pH of 9-13.
3. Magnesium (Mg), titanium (Ti), hafnium (Hf), vanadium (V) and bismuth (Bi) are extremely resistant to neutral and alkaline solutions (strong pH), and high-speed corrosion occurs at low pH (in acidic solutions).
4. In acidic and neutral solutions, molybdenum (Mo), tungsten (W) and rhenium (Re) are resistant to corrosion, but they will be corroded in alkaline solutions.
5. Beryllium (Be), aluminum (Al), copper (Cu), zinc (zinc), cadmium (Cd), tin (Sn), lead (Pb), cobalt (Co), nickel (Ni), zirconium (Zr), gallium (Ga) and indium (in). The last group is the big one. In both acidic and alkaline liquids, they corrode, so they are called amphoteric metals. Only neutral solutions can resist these metals.
Concluding comments
Corrosion can be a frightening experience, and when applied to industries that rely on uncorroded metals to operate, it can lead to some unpleasant effects that are not always recognized: lack of production, injuries, and significant economic losses. Every year, industries lose billions of dollars from their business, mostly due to corrosion. The total cost of global corrosion is enormous; That adds up to about $2.5 trillion, a figure that does not include human protection or environmental consequences.
In continuous industrial practice, tantalum metal has been found to have good corrosion resistance, where the application of tantalum coating can minimize corrosion costs, and it is estimated that a reduction of $875 billion (about) per year is easily achieved.