Morphological structure, lattice system and composition
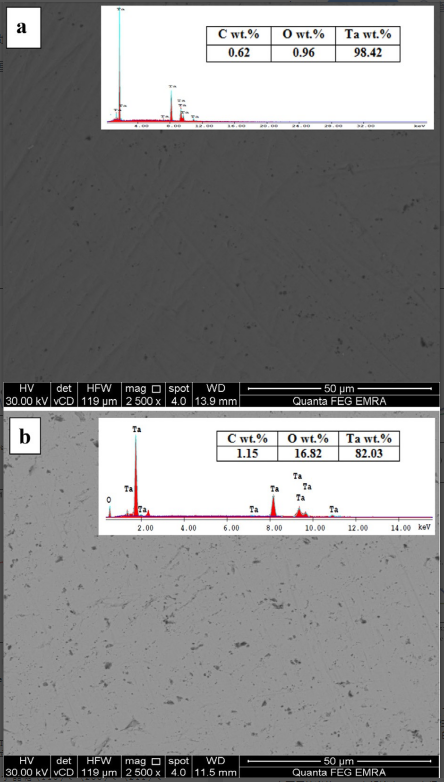
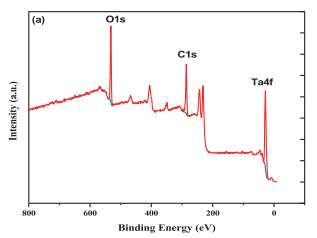
Fig. 3. (a) XPS measurement spectrum of Ta in H2SO4 electrolyte for 6 hours, and (b) high-resolution XPS scanning of Ta4f and (c) O 1s.
Electroanalysis of hydrogen on tantalum electrode
Cathodic polarization linear sweep voltammetry (LSV) was used to study the electrochemical hydrogen evolution of tantalum in different concentrations of H2SO4 electrolyte, and the scanning rate was 5 mV S1. In addition, the apparent activation energy of HER electrocatalyst on tantalum was obtained under different cathodic polarization potentials in 0.5 M H2SO4 electrolyte.
l Effect of electrolyte concentration
In H2SO4 electrolytes with different concentrations, the electrocatalytic activity of Ta electrode for HER was estimated by scanning the cathode LS voltammogram at 25°C in the potential range of 0.0 to 500 mV, as shown in Figure 4. The results show that the cathode current density (ic) of the heat exchanger (that is, the rate of H2 generation in cm2) is significantly dependent on the concentration of H2 SO4 electrolyte, and the tantalum cathode has a high ic value for the heat exchanger. With the increase of H2SO4 electrolyte concentration, the cathode current density of HER on tantalum increases.
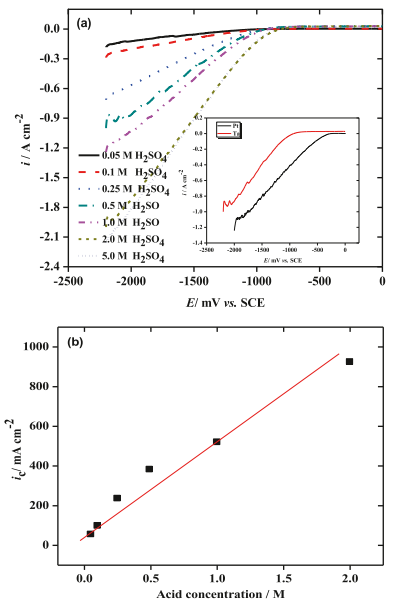
Figure 4. Its performance: (a) Cathodic polarization curves of tantalum electrodes immersed in H2SO4 solutions with different concentrations at 25 C. Illustration, Cathodic thermal decomposition of platinum and tantalum in 0.5 M H2SO4 at 25 C. (b) Cathode current density (1500 mV) and acid concentration.
The inset of fig. 4 shows that the ca- cathode LSV scans tantalum and platinum electrodes in 0.5 M H2SO4 electrolyte at a scanning rate of 5mV S1. The steady-state open-circuit potentials (OCP) of Ta and Pt electrodes are 663 and +439 mV, respectively (see Figure 5). In 0.5 M H2SO4 electrolyte, we can see that in order to achieve the same current density, for example, IC = 100ma cm-2, the HER (ɳH) overpotentials on Ta and Pt cathodes are 520 and 940 mV, respectively. This proves that Ta is a promising candidate cathode for HER in H2SO4 electrolyte.
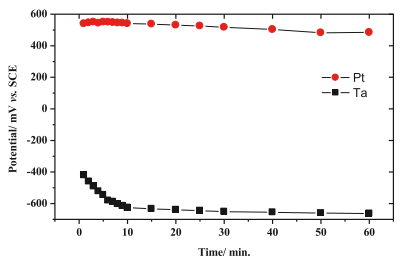
Fig. 5. Changes of open-circuit potentials (OCP, vs SCE) of Pt (●) and Ta (□) electrodes in 0.5 M H2SO4 electrolyte at 25 C with time.
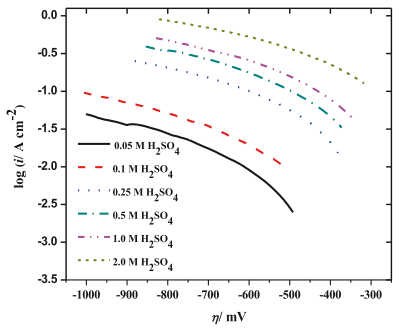
Fig. 6. Tafel diagram of tantalum electrode in different concentrations of H2 SO4 at 25 C.
Fig. 6 shows the cathode Tafel diagram of HER recorded on the ta electrode in H2SO4 electrolyte with different concentrations at 25 c. Tafel slope (b) can be calculated according to the best linear fitting of the measured potentiodynamic polarization curve. According to the relation b = 2.303RT/(1- α)F, the Tafel slope, which is inversely proportional to the cathode transfer Coecient (1-α), is considered as an important parameter to define the rate-determining step (rds) of HER, the dynamics of the electrode process and the inherent properties of the cathode.
According to the data listed in Table 1, the cathodic transfer coefficient (1- α) decreases with the increase of H2SO4 concentration in the electrolyte. When tantalum cathode is used, the value of exchange current density (io) increases with the increase of acid concentration.
The data collected in Table 2 confirmed that our tantalum electrode had good electrocatalytic activity for HER in H2SO4 electrolyte.
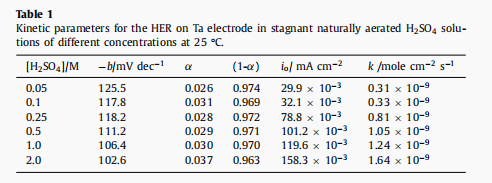
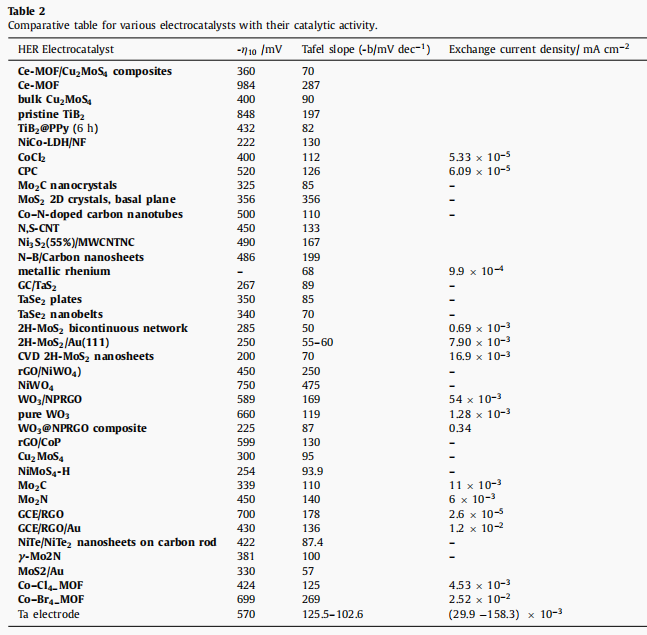
l HER activation energy
The influence of electrolyte temperature on the H2 precipitation rate of Ta catalyst cathode was investigated by measuring the cathodic polarization curve in sulfuric acid electrolyte. In fact, it is very important to study the catalytic activation of HER by electrocatalysts at different temperatures for determining the apparent activation energy (Ea) needed to predict HER mechanism.
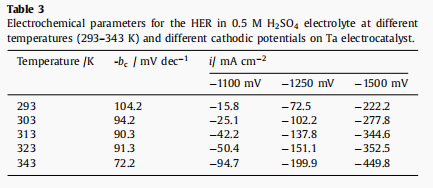
Therefore, 0.5 M H2SO4 solution was selected as the test electrolyte, in which the rate of H2 production on the Ta electrode developed at a considerable rate. Fig. 7a shows a set of HER polarization curves recorded on tantalum substrate at various electrolyte temperatures in the range of 293–343 K.. The corresponding Tafel curve is shown in Figure 7a and its estimated Tafel slope value is given in Table 3 as a function of electrolyte temperature.
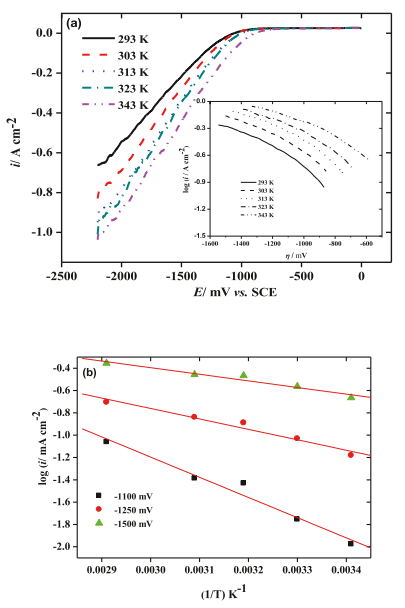
Fig. 7.(a) In a static naturally aerated 0.5 M H2SO4 solution, the cathode on the Ta electrode evolved hydrogen at different temperatures, and the scanning rate was 10mv S1. Illustration: Cathode Tafel line on tantalum electrode immersed in static and naturally ventilated 0.5 M H2 SO4 solution at a scanning rate of 25°C and 5mV S1. Arrhenius diagram For the 0.5 M H2SO4 solution of cathodic hydrogen evolution on the Ta electrode in stagnant natural gas charging, the scanning rate is 5mV s-1, with different polarization potentials, 1100 mV (□), 1250 mV (●) and 1500 mV (▲).
Table 3 with the increase of electrolyte temperature, bc value decreased from 104.2mV dec-1 at 293 K to 72.2mV dec-1 at 343 k. This revealed that HER on tantalum cathode changed from Volmer mechanism to Heyrovsky mechanism, which enhanced the electrocatalytic activity of tantalum on HER.
Study on electrochemical impedance spectroscopy
In order to obtain additional evidence of electrocatalytic activity of tantalum cathode for helium, electrochemical impedance spectroscopy (EIS) was studied.
Fig. 8 clearly shows that the properties of tantalum cathodic impedance diagram are similar for all solutions.
Table 4 shows the fitted impedance pa- Ta electrocatalyst parameters at different H2SO4 concentrations at a fixed operating cathode potential of 1100 mV (relative to SCE).
It may not be necessary to use constant phase element (CPE) to illustrate the electrode/electrolyte interface as a substitute for an ideal capacitor. For comparison, EIS data of ta electrode in 0.5 M H2SO4 electrolyte (OCP) and 1000 mV cathode potential (relative to SCE) were also recorded, as shown in Figure 9.
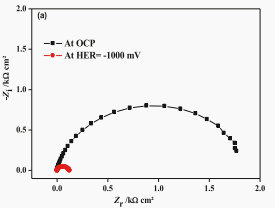
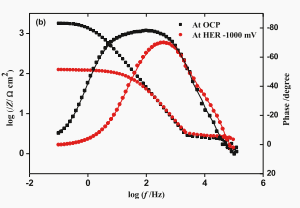
Fig. 9. The impedance spectrum is (a) Nyquist diagram and (b) Bode diagram of tantalum electrode OCP and 0.5 M H2SO4 electrolyte at 1000 mV (relative to SCE) cathode potential.
conclusion
Tantalum is considered as a promising electrocatalyst cathode in sulfuric acid electrolyte. The results of polarization and impedance show that, compared with other eCient-Hull catalysts, hydrogen generation occurs at a reasonable overpotential (H). Among them, the H of helium on tantalum required to reach a current density of 100ma cm-2 is estimated to be 520 mV, while that on platinum is 940 mV, which proves that tantalum can be used as the cathode for the production of green H2 in H2SO4 electrolyte, which is related to the most active electrocatalysis of helium, precious metal Pt. In addition, the activation energy of electrocatalytic HER on the Ta cathode is low (11.5 kJ mol-1 at 1500 mV in 0.5 M H2 SO4 electrolyte), and the evolution of H2 develops on the Ta surface through the single electron transfer step. The ideal combination of lower charge transfer resistance and higher electric double layer capacitance obtained from EIS data makes crystalline ta the best candidate among other electrocatalysts reported so far. HER on tantalum electrode is carried out by Volmer-Heyrovsky reaction mechanism. In the production of green hydrogen fuel, metallic tantalum is considered as the best material for safety, stability and sustainability.